Industry Insights on Implementing GMP-Compliant NGS-Based Workflows
October 15, 2024
Raed Hmadi
The development of next-generation therapeutics, including cell and gene therapies, requires comprehensive evaluation of critical quality attributes (CQAs), such as biosafety, integrity, and potency. The potential of using next-generation sequencing (NGS) assays as an unbiased and highly sensitive technology is becoming increasingly recognized by biopharma organizations. In light of the new regulatory guidelines recommending the adoption of NGS-based assays to replace in vitro and in vivo assays, there is a growing need for digital, in-house end-to-end solutions for NGS data analysis and management, particularly in a validated environment.
Genedata Selector is committed to supporting biopharma organizations in digitalizing and streamlining NGS-based workflows to accelerate time to market. By bringing together experts from leading biopharma organizations, Genedata Selector facilitates community building and knowledge exchange, empowering R&D scientists at different stages to understand the role of GMP-compliant NGS-based workflows in advancing the development and manufacturing of therapeutics.
10th Genedata Selector Open Forum
On October 10, 2024, Genedata Selector hosted its 10th Open Forum with industry experts from 35+ biopharma organizations. The virtual event focused on the adoption of NGS-based workflows, particularly long-read sequencing assays, from the early stages of the R&D process and method development to the later stages of implementation in validated environments in accordance with regulatory compliance. After a warm welcome, Thomas Hartsch, Head of Business at Genedata Selector, shared a brief history of the Open Forum and highlighted the significance of the 10th event, which shortly followed the landmark release a few months earlier of Genedata Selector 10. The latest release features a new generation of Playbooks, powered by Nextflow pipelines, to support NGS-based assays in GMP environments. Thomas also emphasized the current regulatory guideline updates, including the ICH Q5A(R2) guideline that came into effect in 2024.
Participant Testimonials
Bristol Myers Squibb
Krishnamurthy Shankarling, Senior Principal Scientist at Bristol Myers Squibb, kicked off the Open Forum by showcasing the role of Genedata Selector Playbooks in automating omics data management and analysis workflows. He presented the parameters required to characterize AAV particles for gene therapy development and discussed how both short- and long-read sequencing technologies are used in such workflows. Krishnamurthy emphasized the ease and efficiency of using Genedata Selector Playbooks, highlighting their significant role in streamlining AAV-based gene therapy and their strong potential to continue supporting such analyses in the future.
Genedata Selector
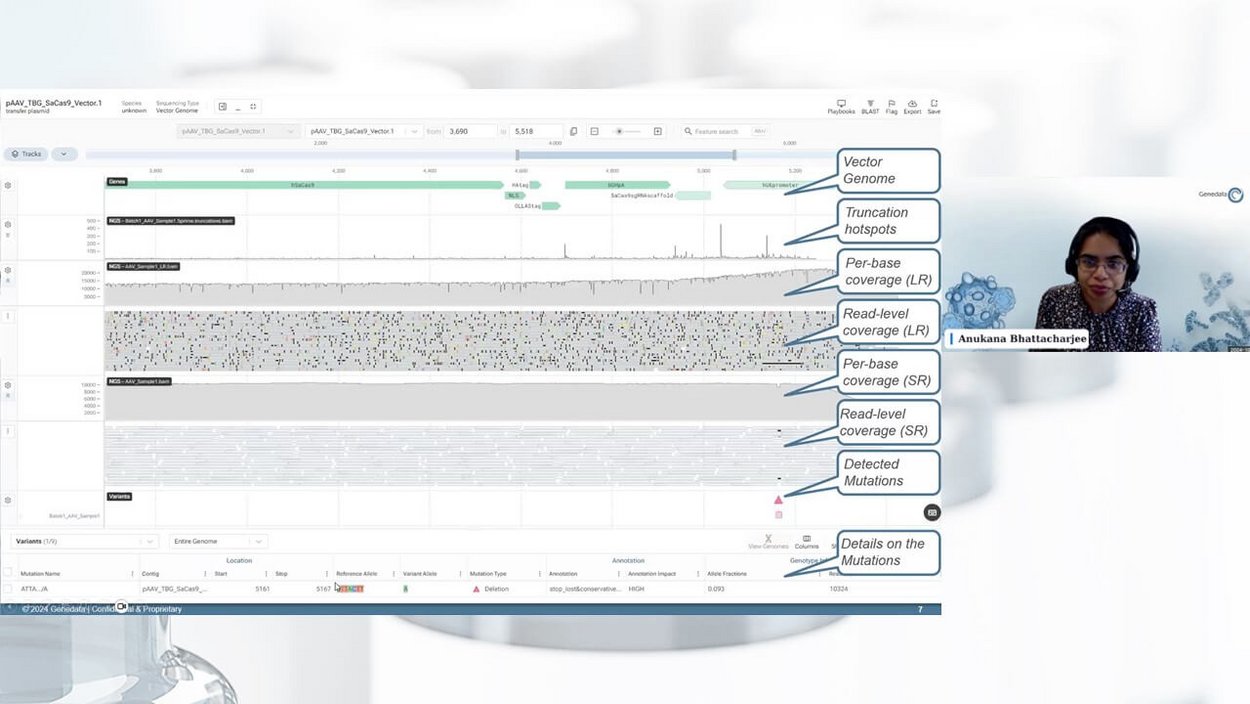
Anukana Bhattacharjee, Scientific Consultant at Genedata Selector, demonstrated how Genedata Selector supports NGS-based assays utilizing long-read sequencing for the quality control of AAV vectors. She presented Genedata Selector Playbooks, which enable R&D scientists to easily investigate full-length genome sequencing datasets, detect both structural and low-frequency variants, and characterize complex rearrangements and truncation hotspots. Through a live demo using standardized and customized Playbooks, Anukana showcased how the complex analysis of NGS datasets can be transformed into automated and streamlined workflows, significantly improving efficiency and decision-making during AAV-based gene therapy development.
Fujifilm Dionsynth
Andrew Hillhouse, Principal Scientist Group Lead at Fujifilm Diosynth Biotechnologies, presented his team’s work on developing workflows for viral and plasmid identification using long-read sequencing assays. Using Genedata Selector software, the team streamlined base calling workflows, reducing analysis time from 61 hours to 4 hours — a 90% time saving. A specially developed Playbook also detected contaminants, resulting in over 99% of sequencing reads being accurately identified and mapped to the reference genome. Andrew concluded by highlighting the potential of long-read sequencing technologies and the successful deployment of Genedata Selector at Fujifilm.
Astellas Institute for Regenerative Medicine
Tracie Fradet, Associate Director of Cytogenomics at the Astellas Institute for Regenerative Medicine (AIRM), concluded the first half of the Open Forum with a presentation about her group’s effort to develop a GMP-compliant NGS workflow that meets evolving regulatory requirements. She explained the key elements necessary for developing an efficient and automated NGS workflow to support successful regulatory submissions. Tracie highlighted the value of Genedata Selector in facilitating the integration, analysis, and sharing of project-related data in a GMP environment, thereby supporting data-driven decision-making. Additionally, she shared insights from a recent FDA Type D meeting held at AIRM, where the FDA expressed their alignment with AIRM’s NGS workflow and validation approach.
Panel Discussion
The second half of the Open Forum brought together all the speakers for a roundtable discussion on leveraging NGS-based workflows to streamline product QC at various stages of the R&D process. The panelists addressed the challenges of developing scalable development and manufacturing processes and how automated, compliant NGS-based workflows can help overcome these obstacles. They emphasized the role of different NGS technologies and the power of end-to-end digital solutions in streamlining analysis workflows, supporting R&D scientists with reliable, data-driven decision-making.
The panelists emphasized the importance of time and safety in the development and manufacturing of CGTs, as well as economic viability. Robust processes with the right digital infrastructure are therefore required to streamline and optimize these workflows. Based on data from in-house projects, panelists highlighted how the speed and efficiency of the Genedata Selector platform helped to accelerate timelines and overcome analysis bottlenecks when assessing multiple CQAs of CGTs. Genedata's end-to-end digital analysis solution enables all team members to minimize human error, run multiple analyses in parallel, and generate GMP-compliant reports for internal evaluation and regulatory submissions.
Discussions with regulatory agencies, such as the FDA, on how to handle the analysis of generated NGS datasets and ensure their integrity were also addressed. All panelists agreed that early collaborations with regulatory agencies is critical to accelerate development timelines and ensure positive outcomes. In addition, Astellas also thanked the Genedata Selector QA team for their support to AIRM throughout the validation process and during the preparation for their Type D meeting with the FDA.
Summary
In summary, the 10th Genedata Selector Open Forum further highlighted the impact of automating NGS-based workflows and on ensuring regulatory compliance. If you would like to hear more about Genedata Selector, you can connect with our team at GTAD, CTAD and IABS.
To join our unique community and stay informed about the latest developments in automating compliant NGS workflows, register by clicking the button below:



